The ocean and the atmosphere are constantly seeking balance.
Gases like oxygen, nitrogen, and carbon move between the ocean’s surface and the atmosphere by billions of metric tons every year.
A higher concentration of one gas in the atmosphere leads to more of that gas being taken up by the ocean as the two try to reach a state of balance – or equilibrium. However higher concentrations of carbon, emitted by human activities predominantly through the burning of fossil fuels, have been observed in the atmosphere since the Industrial Revolution.
This has consequently led to an increase in the ocean’s accumulation of carbon globally. This increase in ocean carbon has caused a chain of chemical reactions driving one of the primary environmental threats to marine ecosystems, fisheries and coastal communities – ocean acidification.

Carbon dioxide absorbed at the ocean’s surface binds with water molecules to produce an acid known as carbonic acid (H2CO3), which dissociates into bicarbonate ions (HCO3–) and a free-floating hydrogen ion (H+).
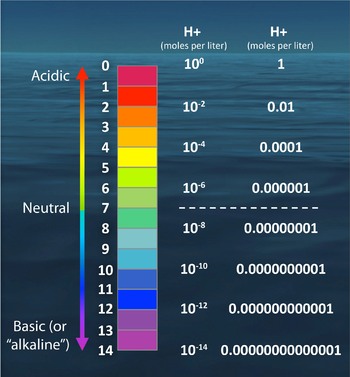
A rise in hydrogen ions is what changes the pH of any liquid, lowering the pH and making it more acidic.
The ocean’s absorption of carbon dioxide from the atmosphere is a natural process that has occurred over Earth’s history. However, today with more carbon in the atmosphere globally, we also see more carbon in the surface ocean that is drawing down the surface ocean’s pH and leading to global ocean acidification.
The ocean’s pH at the sea surface once measured at 8.2 before the 1700’s. Today, it’s estimated at 8.1.
So while, 0.1 decrease in ocean pH may seem insignificant, it means the concentrations of hydrogen ions within the ocean have increased dramatically. The pH scale is logarithmic, meaning that each whole number change on the scale represents a tenfold change in acidity or basicity.
In human blood, a 0.1 decrease in pH can lead to seizures, heart arrhythmia or even a coma. Here, we’re talking about a 0.1 pH decrease across the global surface ocean – a body of water that spans 139 million square miles.
The 0.1 decrease in global ocean pH means our oceans have increased in acidity by 25% in the last 300 years. And with continued growth in atmospheric carbon emissions, this trend will continue.
Based on model simulations scientists estimate that by 2100, the average pH of the ocean’s surface waters could decrease by 0.3 – 0.4. This is equal to a 100% to 150% increase in acidification over the next 75 years.
Scientists are already observing the impacts of a lower pH ocean. Increasingly acidic waters erode away at the calcium carbonate shells and skeletons of scallops, crabs, oysters, clams, mussels and entire coral reefs.
In the Pacific Northwest, it has already impacted oyster farms with mass die-offs. NOAA-funded studies have found ocean acidification is leading to the dissolution of shells and sensory organs of young Dungeness crabs, a crustacean supporting the most valuable fishery on the U.S. West Coast – valued between $77 to $200 million annually for the 2007/2008 to 2019/2020 seasons.
In particular, juvenile shellfish such as Atlantic sea scallops, are generally even more susceptible to low pH conditions, making it difficult for them to grow into mature adults. Atlantic sea scallops support one of the most valuable fisheries in the United States, worth $670 million in 2021.
It accelerates the erosion of coral reefs and decreases their ability to grow (i.e. calcify), leading to the loss of critical infrastructure for storm surge protection and one of the Earth’s most crucial habitats providing shelter to a vast breadth of marine biodiversity.
However, the impacts of ocean acidification are not consistent across the ocean. In some places, it’s exacerbated. In other regions, ocean acidification is being buffered against by complex changes in seawater carbonate chemistry.

Therefore, better observing regional changes related to ocean acidification, seeking long-term solutions and mitigating the effects on key ecosystems is critically important to mitigating the effects of ocean acidification. This is a big job for scientists today who are working hard to identify the intensity and the ecosystem effects of ocean acidification that we can expect in the future. They ask questions like – how does it vary across entire regions, how will it intensify over years and decades, and what ecosystems are most vulnerable?
Scientists at AOML are doing exactly that with research cruises, the deployment of cutting-edge instruments, and advanced modeling efforts.
Research Cruises
Identifying the impacts of ocean acidification across vastly different ecosystems requires a comprehensive view and long term monitoring of changes in ocean chemistry across thousands of miles of U.S. coastline.
To do this, scientists at AOML lead the Gulf and Ocean Monitoring Ecosystems and Carbon Cruises (GOMECC) and East Coast Ocean Acidification (ECOA) cruises every four years to assess changes in seawater chemistry across the Gulf region and greater south Atlantic.

By deploying a CTD at specified stations from offshore to shallow waters along the coast, scientists across institutions collect seawater samples at different depths to holistically assess the partial pressure of carbon dioxide (pCO2) – an indication of how much carbon dioxide is in seawater.
With these cruises returning to specified sites every four years, scientists can assess long-term changes in the seawater chemistry and identify the regions most impacted by increasingly acidic seawater.
In South Florida, scientists at AOML with the Ecosystem Assessment team perform seven-day South Florida Ecosystem Restoration Program (SFER) cruises from Biscayne Bay to the Florida Keys and up along the West Florida Shelf as far north as Tampa Bay collecting profiles of dissolved inorganic carbon and total alkalinity – key parameters used to investigate ocean carbonate system chemistry across depth.
By returning to designated cruise paths (i.e. transects) with marked coordinates every other month, the team has measured changes in carbonate chemistry across the Florida Keys over seasons, years and decades.



Deploying Instruments
The Argo Program is currently working to seed the global ocean with robotic ocean profiling floats that can observe ocean pH from the sea surface to 2,000 meters. This program will revolutionize ocean acidification observing by providing, for the first time, a global scale snap shot of real-time global ocean pH conditions. These observations will compliment the highest quality measurements made by scientists on research cruises and fill major observing gaps that have existed in the past. At AOML, researchers have deployed the first Biogeochemical-Argo floats in the Gulf of America, and have been collecting real-time pH observations within the region. The Gulf is home to numerous ecologically and economically important marine species, many of which are vulnerable to the present shifts in ocean pH that are being observed.

Fully comprehending the global effects of ocean carbon uptake also requires understanding the process by which dissolved carbon in the sunlit surface ocean is transformed by phytoplankton and transported to the deep ocean in the form of biological debris known as “marine snow.” The delivery of marine snow to the seafloor where it can be preserved for long periods is the primary pathway that the ocean is able to sequester carbon for decades to millennia.
Known as the biological pump, any changes in the strength of this process would influence the ocean’s ability to accumulate atmospheric carbon and it is currently unknown how warming temperatures and declining pH within the surface ocean may affect this process in the future.
To investigate this process, scientists at AOML deploy a complex sediment trap up to 1,100 meters deep in the Gulf of America region to collect and investigate the microscopic life comprising this marine snow and driving the transport of carbon to the deep.
By examining the fossilized, microscopic shells of one key type of zooplankton, formanifera, scientists at AOML and the US Geological Survey (USGS) are also unlocking crucial new insights into ocean chemistry and the growth of small shelled zooplankton that play a role globally in the biological carbon pump.



Monitoring fluctuations in the ocean’s pH in real time is also crucial. Within the Florida Keys National Marine Sanctuary (FKNMS), Sofar “Spotter” buoys are deployed at vital Mission: Iconic Reef sites to measure wave energy, wind speed, sea surface temperature, and pressure near the surface. Below the surface, integrated sensors measure pH and seafloor temperature in near-real time – actively monitoring ocean acidification on crucial reefs.

Supporting the ambitious NOAA-wide Mission: Iconic Reefs effort, deploying these novel integrated systems is the beginning of an easily – scalable solution to monitor marine environments and fill gaps in ocean acidification research by providing better data collection both spatially and temporally.
Modeling
On coral reefs, the wild variety of life from seagrass beds to corals to algae influences seawater chemistry – and the localized impacts of ocean acidification. As they perform metabolic processes such as photosynthesis, respiration and calcification, they naturally change the levels of dissolved inorganic carbon (DIC) within the seawater – leading to changes in the local pH.
With a new study, scientists at AOML have developed a statistical model that uses water mass histories to trace how interactions with upstream ecosystems metabolically modify seawater chemistry, improving predictions of the chemical conditions experienced by coral reefs. .
With the long-term dataset produced by the South Florida Ecosystem Restoration (SFER) cruises collecting carbonate chemistry measurements, scientists can train the model to predict chemical changes across miles of a barrier reef.
By building and validating the model now, scientists hope to apply it to future predictions of how exacerbated ocean acidification could impact carbonate chemistry across the only barrier reef in the continental United States.
Benthic communities (i.e. seagrass, coral), source water (“endmember”) chemistry and the complex flow of water (hydrodynamics) between habitats all influence the local carbonate chemistry of a coral reef. Derived from: Hirsh, et al., 2025
Ocean acidification is a global stressor. By investigating the ocean’s accumulation of carbon, the biological pump, localized changes in seawater chemistry across seasons and years, and modeling these changes, scientists at AOML can assess how ocean acidification will impact the key ecosystems we depend on most.
