Steven M. Davis, South Florida Water
Management District, West Palm Beach, Fl.
A brackish water
ecotone of coastal bays and lakes, mangrove and buttonwood forests, salt
marshes, and tidal creeks separates Florida Bay from the freshwater
Everglades. The 24 km-wide ecotone
adjoins the north shoreline of Florida Bay between Highway Creek (US1) and
Whitewater Bay, which delineate the eastern and western boundaries of the
mangrove estuary transition model.
Whitewater Bay is included in the model because it is influenced by the
Shark River drainage basin originating in the Everglades. The mangrove estuary transition is
characterized by a salinity gradient and mosaic that vary spatially with
topography and that vary seasonally and inter-annually with rainfall and
freshwater flow from the Everglades.
Because of its location at the lower end of the Everglades drainage
basin, the mangrove estuary transition zone is potentially affected by upstream
water management practices that alter the freshwater heads and flows that drive
salinity gradients.
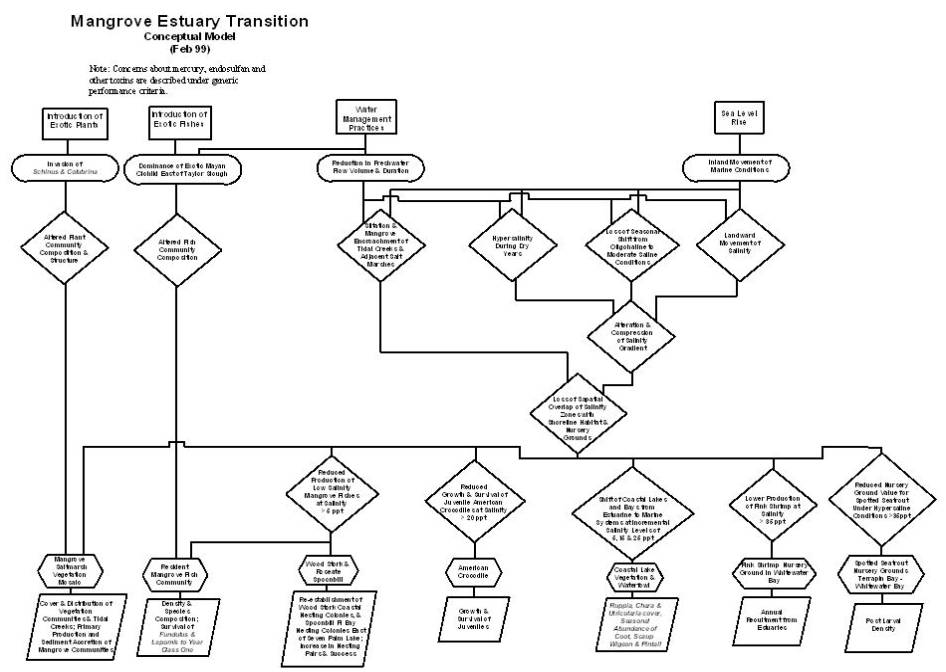
Stressors on the
mangrove estuary transition ecosystem and the drivers that create them fall
into five categories (refer to figures on pages D-A-87& 88). Sea level rise is an important non-societal
driver (Wanless et al., 1994) that is causing the inland movement of marine
conditions into the estuary transition zone (Meeder et al., 1996). The inland movement of marine conditions due
to sea level rise is happening independent from other societal-driven
stressors. The societal-driven water
management operations of the C&SF Project stress the transition zone
through reductions in the volume and duration of freshwater flow
entering the zone (McIvor et al., 1994)
The introductions of exotic fishes and plants are other societal-driven
drivers that have resulted in the dominance of the Mayan cichlid east of
Taylor Slough (Trexler et al., in prep.) and the invasion of Schinus
and Colubrina
into mangrove forests (Armentano et al., 1995). The societal input and bioaccumulation of mercury and other
toxins pose a threat to faunal health at all trophic levels in all south
Florida ecosystems, including the mangrove estuary transition, as discussed
under generic issues.
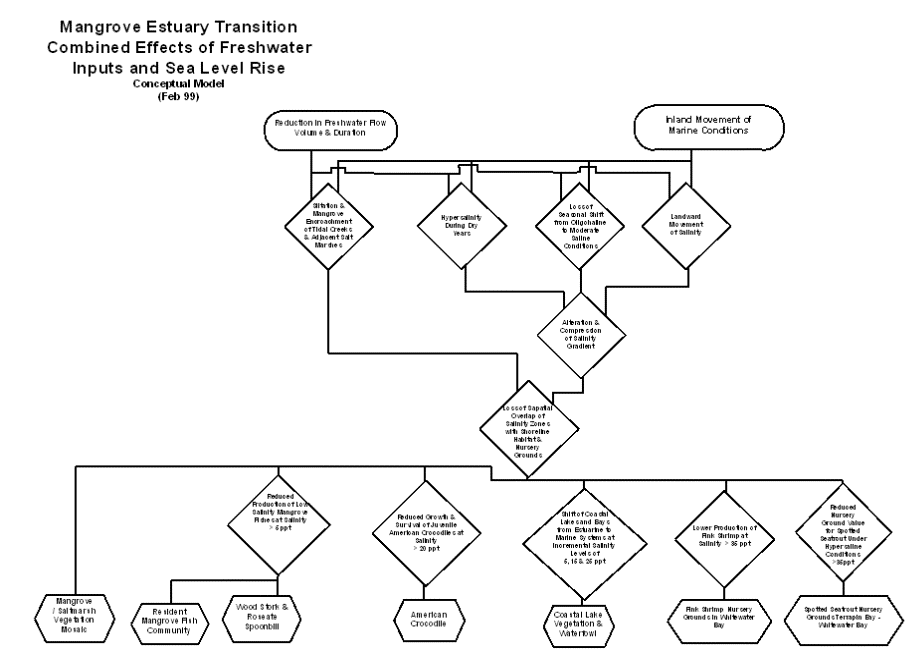
The inland movement
of marine conditions due to sea level rise and the reduced input of freshwater
due to water management work together to alter and compress the salinity
gradient of the mangrove estuary transition (Browder and Moore, 1981). They both result in the landward movement of
salinity, the loss of a seasonal shift from moderate saline to oligohaline
conditions, and occurrence of hypersalinity within the transition zone during
dry years. Both directly change habitat structure by contributing to the
siltation and mangrove encroachment of tidal creeks (Meeder et al., 1996), to
the extent that open water courses that were described earlier this century are
no longer recognizable (Glen Simmons, personal communication).
The alteration and
compression of the salinity gradient results in the loss of the spatial overlap
of salinity zones with shoreline habitat and nursery grounds (Browder and
Moore, 1981). Ecological values of the
mangrove estuary transition that depend on the overlap of salinity and habitat
include the mangrove/salt marsh vegetation mosaic, the resident mangrove fish
assemblage, the wood stork and roseate spoonbill, the American crocodile,
spotted seatrout nursery grounds, pink shrimp nursery grounds, and coastal lake
vegetation and waterfowl.
Mangrove/salt marsh vegetation mosaic.
The alteration and compression of the salinity gradient potentially can
affect the community cover, distribution, and production of the mangrove
forests (Rhizophora,
Avicennia,
Laguncularia,
and Conocarpus),
salt marshes and tidal creeks of the mangrove estuary transition that are
documented by Welsh et al. (1995). Some
mangrove forests in the transition zone have experienced invasion by the exotic
trees Schinus
and Colubrina
(Armentano et al., 1995).
Tidal creeks and adjacent salt marshes have been encroached by red
mangrove as described above. The
invasion of the freshwater marl marshes at the upstream end of the salinity
gradient by red mangrove (Meeder et al., 1996) corresponds to an accelerated
rate of sea level rise. The vegetation
mosaic defines the habitats of the mangrove estuary transition zone. The spatial distribution of those habitats,
in combination with the salinity gradient that overlays them, may determine the
suitability of this region to sustain its ecological attributes (Browder and
Moore, 1981). The importance of this
habitat mosaic warrants the monitoring of the distribution and cover of
vegetation communities and tidal creeks in the mangrove estuary transition
zone as efforts proceed to restore freshwater inputs and salinity regimes.
Resident mangrove fish community.
The resident fish community of sheepshead, sailfin mollies, topminnows,
rainwater killifish, and sunfish thrives under low salinity, decreasing in
production and increasing in mortality when salinity exceeds 5-8 ppt (Lorenz,
1997 and in press). The exotic Mayan
cichlid has become established in this fish community to the extent that it
presently is the dominant species from Taylor River east to Highway Creek
(Trexler et al., in prep.). Lowered
salinity regimes due to increased freshwater inputs are expected to result in
community recovery as measured by increased production and abundance of
resident mangrove fishes. The
dependence of wood storks on larger resident mangrove fishes above
approximately 10 cm in length (Ogden et al., 1978) provides an additional
measure of increased survival of topminnows and sunfish to year class one
(into their second year of life).
Consistently lower salinities at the upstream end of the salinity
zonation are expected to reduce mortality and allow survivorship of these
species to the larger size classes that are available to wood storks.
Wood stork and roseate spoonbill.
The collapse of the coastal nesting colonies of wood storks and great
egrets is attributed largely to a decline in the production and density of the
resident mangrove fishes (Ogden, 1994), particularly topminnows and sunfish
that survive past their first year to a size that wood storks can capture
(Ogden et al., 1978). The decline in
roseate spoonbill nesting and the shift of nesting distribution from eastern to
western Florida Bay (Powell et al., 1989) are also attributed to the reduction
in populations of resident mangrove fishes upon which they feed (Bjork and
Powell, 1994). Small fishes have been
reported to be the primary part of the diet of roseate spoonbills in Florida
Bay (Allen, 1942; Powell and Bjork, 1990).
Increased density of resident mangrove fishes and increased fish
survival to year class one, as a result of consistently lower salinity patterns
at the upstream end of the gradient, are expected to contribute to the re-establishment
of wood stork coastal nesting colonies, the re-establishment of roseate
spoonbill Florida Bay nesting colonies east of Seven Palm Lake, and an increase
in number of nesting pairs and nesting success of both species.
American crocodile.
The American crocodile dwells in the ponds and creeks of the mangrove
estuaries of Florida Bay (Ogden, 1976; Mazzotti, 1983). American crocodiles are tolerant of a wide
salinity range as adults because of their ability to osmoregulate (Mazzotti,
1989). Juvenile crocodiles lack this
ability, however, (Mazzotti, 1989) and their growth and survival decline at
salinities exceeding 20 ppt (Mazzotti et al., 1988; Mazzotti and Dunson, 1984;
Moler, 1991). Juvenile crocodiles tend to seek freshwater pockets such as black
mangrove stands when those choices are available. Re-establishment of a salinity gradient with levels below 20 ppt
in shoreline and tidal creek habitats, which would indicate a gradient and
mosaic of lower salinities upstream, is expected to benefit the crocodile as
measured by increased growth and survival of juveniles.
Spotted sea trout nursery grounds.
Post larval spotted sea trout utilize the coastal basins of the Florida
Bay mangrove estuary as nursery grounds from Terrapin Bay west to Whitewater
Bay. Densities of post larvae in those
basins are highest at an intermediate salinity range of 20-30 ppt, and
densities drop when salinity exceeds that of seawater (35 ppt) (Thayer et al.,
1998; Schmidt, 1993). Restoration of a
salinity gradient with a persistent zone of <35 ppt in the coastal basins,
as a result of freshwater input from upstream, is expected to result in an increase
in the post larval density and thereby an enhancement of the nursery ground
value for spotted sea trout and possibly other sport fish species in the
coastal basins.
Pink shrimp.
Mangrove estuaries in Everglades National Park, along with Florida Bay,
are nursery grounds for pink shrimp, an ecologically and economically important
species in south Florida. Pink shrimp
are harvested commercially on the Tortugas grounds, and the pink shrimp fishery
is one of south Florida’s most valuable fisheries in terms of ex-vessel value. Pink shrimp are also a food source for many
recreationally and commercially important estuarine and marine species such as
mangrove snapper and spotted seatrout (Higher Trophic Levels Working Group,
1998).
Pink Shrimp
spawning occurs in the Dry Tortugas area, and eggs and larvae are carried
inshore by currents and tides (Jones et al., 1970; Hughes, 1969). Browder (1985) and Sheridan (1996) have
found positive relationships between indices of freshwater inflow to the coast
and Tortugas pink shrimp landings.
Sheridan’s annually updated statistical model based on various
freshwater inflow indices has successfully predicted annual pink shrimp
landings in most of the past decade (Sheridan 1996 and unpublished). The salinity gradient associated with
coastal runoff may provide navigational directions to immigrating young pink
shrimp (Hughes, 1969). Survival rates
of juvenile pink shrimp are sensitive to salinity and decrease markedly under
extreme hypersaline conditions (Browder, in press). Optimal salinities for survival are not fully determined, but
probably are somewhat below that of seawater (35 ppt). Tabb et al. (1962), Rice (1997), and others
have documented that the mangrove estuaries in the Whitewater Bay system of
Everglades National Park are pink shrimp nursery grounds.
Coastal lake vegetation and waterfowl.
Compression of the salinity gradient has changed the coastal lakes and
basins of the mangrove ecotone from estuarine to predominantly marine
systems. Coastal lakes such as Seven
Palm Lake, Cuthburt Lake, Long Lake, West Lake, Lake Monroe and the Taylor River
ponds are contained within the mangrove forest and are connected to Florida Bay
only by tidal creeks. Coastal basins
such as Joe Bay, Little Madeira Bay, Terrapin Bay, Garfield Bight, and
Whitewater Bay open directly to Florida Bay or the Gulf of Mexico. The coastal lake and basin estuary
ecosystems require seasonal salinity variations from oligohaline (wet season)
to mesohaline (dry season) conditions, in contrast to the mesohaline to marine
conditions that presently occur during most years. Prolonged periods of
salinity concentrations near that of seawater (35 ppt) in the coastal lakes and
basins appear to have contributed to the near-elimination the once-abundant
beds of the submerged aquatic plants Ruppia, Chara, and Utricularia
(Ogden, personnal communication) which require oligohaline to mesohaline
conditions (Morrison and Bean, 1997). Utricularia
tolerates only oligohaline salinities with an upper limit of 5-8 ppt. Chara also thrives under freshwater
conditions but tolerates mesohaline salinities up to 15-20 ppt. Ruppia grows under a mesohaline salinity
range of 10-25 ppt. Waterfowl species
including coot, scaup, widgeon and pintail feed on the Ruppia, Chara, and Utricularia. The reduction in beds of these plants
apparently has contributed to the precipitous decline in numbers of seasonally
abundant waterfowl that formerly utilized the coastal lakes and basins (Kushlan
et al., 1982). Recent high-rainfall
years have witnessed an increase in coot numbers on the West Lake to
approximately 2000 during winter 96-97 (Bass, personal communication), but not
to the population size of approximately 50,000 that over-wintered there until
the 1960's (Kushlan et al., 1982).
Re-establishment of a salinity gradient that restores seasonal variation
from oligohaline to mesohaline conditions in the coastal lakes and basins is
expected to result in an increase in the aerial cover of Ruppia,
Chara,
and Utricularia
and the return of winter waterfowl populations of coot, scaup, widgeon and
pintail to the lakes and basins.
Ecological restoration of the mangrove estuary transition requires a reduction in the frequency of high salinity events that have been identified for each coastal basin through the conceptual model process. Another restoration measure is to increase the frequency of low salinity events that have been identified for each coastal basin. The high and low salinity levels represent the best professional judgement of those scientists working in the mangrove estuary, based on the existing information on the biological requirements and distributions of the estuarine organisms that are described above, available salinity data, and field observation.
Table 1 displays the lower and upper
salinity levels identified for coastal basins.
It is desirable to decrease the frequency that salinity exceeds upper
levels, and to increase the frequency that salinity drops below lower levels.
Table 1
Salinity Values
|
Basin |
Lower Level |
Upper Level |
|
Joe bay |
5 ppt |
15 ppt |
|
Little Madeira Bay |
15 ppt |
25 ppt |
|
Terrapin Bay |
25 ppt |
35 ppt |
|
Garfield Bight |
25 ppt |
35 ppt |
|
North River Mouth |
5 ppt |
15 ppt |
The strategy for ecological
restoration of the mangrove estuary transition is to maintain freshwater heads
and flows in the Everglades at the upstream end of the salinity gradient in
order to achieve desirable salinity regimes in the Florida Bay coastal basins
at the downstream end of the salinity gradient. Regression analyses demonstrated inverse relationships of
salinity in the coastal basins to water level upstream in the Everglades
(Davis, 1997). The regressions
indicated that stages of 7.3 and 6.3 feet msl at the P33 gage in central Shark
River Slough produce the lower and upper salinity levels for Joe Bay, Little
Madeira Bay, Terrapin Bay, Garfield Bight, and North River Mouth. Four performance measures for the ecological
restoration of the Florida Bay mangrove estuary and coastal basins are derived
from the simulated stages at the P33 gage and salinity levels in the coastal basins.
The frequency of stages of 6.3+ at P33
is applied as a performance measure for the Florida Bay coastal basins. The performance measure is the number of
months during the 31-year period of record when stages at P33 rose to, or
above, 6.3. The target is the number of
months that NSM45F provided stages of 6.3 or above. A reduced frequency of high salinity events is given a high
priority in the ecological restoration of the coastal basins, thus the
frequency of 6.3+ stages is given a weighting of two when averaged with the
other performance measures.
The frequency of stages of 7.3+ at P33
is applied as a performance measure to the Florida Bay coastal basins. The performance measure is the number of
months during the 31-year period of record when stages at P33 rose to, or
above, 7.3. The target is the number of
months that NSM45F provided stages of 7.3 or above. An increased frequency of low salinity events is given a lower
priority than a reduced frequency of high events, thus the frequency of 7.3+ stages
is given a weighting of one when averaged with the other performance measures
for the coastal basins.
The transition from the late dry
season to the early wet season during March through June is a critical period
to estuarine organisms in the Florida Bay coastal basins regarding the
frequency and duration of high salinity events. Salinity is estimated based on
relationships between mean monthly salinity in the coastal basins and water
stage at the P33 gage in mid Shark River Slough. The cumulative salinity difference (ppt) from the high salinity
levels that have been identified for Florida Bay coastal basins is summed
during the dry/wet season transition months of March-June. Differences are summed over five coastal
basins (Joe Bay, Little Madeira Bay, Terrapin Bay, Garfield Bight and North
River Mouth) and over the 31-year period of record. Differences above the specified high salinity levels are given a
positive value, and differences below the high salinity levels are given a
negative value. The target is to reduce
the cumulative salinity difference to a value that does not exceed the
cumulative difference produced by NSM45F.
The cumulative March-June salinity difference from high levels is given
a weighting of one when averaged with the other performance measures for the
coastal basins
During the August-October transition
from the late wet season to the early dry season, it is important to achieve
low salinity levels in the Florida Bay coastal basins to provide the seasonal
environment for low-salinity estuarine organisms and to postpone the onset of
high salinity events further into the dry season. Salinity is estimated based
on relationships between mean monthly salinity in the coastal basins and water
stage at the P33 gage in mid Shark River Slough. The cumulative salinity difference (ppt) from the low salinity
levels that have been identified for the Florida Bay coastal basins is summed
during the wet/dry season transition months of August-October. Differences are summed over the five coastal
basins and over the 31-year period of record.
Differences above the specified low salinity levels are given a positive
value, and differences below the low salinity levels are given a negative
value. The target is to reduce the
cumulative salinity difference to a value that does not exceed the cumulative
difference produced by NSM45F. The
cumulative August-October salinity difference is given a weighting of one when
averaged with the other performance measures for the coastal basins.
Ecological attributes
and indicators of restoration success in the Florida Bay mangrove estuary and
coastal basins that are linked to the above hydrology/salinity performance
measures in the conceptual model include 1) increased production of
low-salinity mangrove fishes, 2) re-establishment of coastal nesting colonies
of wood storks/great egrets and eastern Florida Bay colonies of roseate
spoonbill, 3) earlier timing of coastal colony formation by wood storks/great
egrets and of Florida Bay colony formation by roseate spoonbills, 4) increased
growth and survival of juvenile American crocodiles, 5) increased cover of
low-to-moderate salinity aquatic macrophyte communities in coastal lakes and
basins, 6) return of seasonal waterfowl aggregations to coastal lakes and basins,
7) enhanced nursery ground value for spotted seatrout and pink shrimp in
coastal basins, and 8) persistence and resilience of the mangrove, salt marsh
and tidal creek vegetation mosaic.
A performance
measure that is generic to the conceptual models of all physiographic regions
of south Florida is the input and bio-accumulation of mercury and other
toxins. Potential inputs of mercury and
pesticides in agricultural and urban runoff water that may be needed for freshwater
input into the mangrove estuary transition might result in reduced health,
behavioral and physical abnormalities, and loss of reproductive vigor of the
fauna unless measures are taken to restrict loads of these toxins in inflow
water. Measures of faunal health that
reflect responses to mercury and pesticide inputs include body burdens and the
incidence of physical and behavioral abnormalities in representative species.
Predicted rises in
sea level require re-evaluation of relationships between Everglades stage and
mangrove estuary transition salinity during the next century. However, the strategy for the maintenance of
salinity at the lower end of the gradient by adjusting upstream water stage at
key Everglades gages will continue to apply.
Maintaining Everglades stages based on presently derived stage/salinity
relationships provides one potential strategy to support a salinity gradient,
but with a landward shift in response to rising sea level. Raising Everglades stages based upon revised
stage/salinity relationships provides another potential strategy to offset sea
level rise and maintain the mangrove estuary transition in its present
location. Regardless of rising sea level, however, a salinity gradient
supportive of an ecologically functional mangrove estuary transition zone will
be required to maintain the integrity of the south Florida ecosystem.


Allen,
R.P. 1942. The Roseate Spoonbill.
Dover Publications, New York: 142pp.
Armentano,
T.V., R.F. Doren, W.J. Platt and T. Mullins.
1995. Effects of Hurricane
Andrew on coastal and interior forests of southern Florida: Overview and synthesis. Journal of Coastal Research Special Issue 2:
111-114.
Bass,
O.L., Jr. Personal communication. Everglades National Park, Homestead FL.
Bjork,
R.D. and G.V.N. Powell. 1994. Relations between hydrologic conditions and
quality and quantity of foraging habitat for roseate spoonbills and other
wading bird in the C-111 basin.
National Audubon Society Final Report to South Florida Research Center,
Everglades National Park.
Browder,
J.A. 1985. Relationship between pink shrimp production on the Tortugas
grounds and water flow patterns in the Florida Everglades. Bulletin of Marine Science 37: 830-856.
Browder,
J.A. In press. Environmental influences on potential
recruitment of pink shrimp, Penaeus duorarum, from Florida Bay nursery
grounds. Estuaries 1999.
Browder,
J.A. and D. Moore. 1981. A new approach to determining the
quantitative relationship between fishery production and the flow of fresh water
to estuaries. In: R. Cross and D. Williams, eds. Proceedings of the National Symposium on
Freshwater Inflow To Estuaries,Volume 1, FWS/OBS-81/04, Office of Biological
Services, U.S. Fish and Wildlife Service, Washington D.C.: 403-430.
Davis,
S.M. 1997. Salinity in coastal basins estimated from upstream water
stages. In: Central and Southern Florida Project Comprehensive review Study, http://www.restudy.org, Comprehensive Plan
Evaluation, Evaluation of Alternative Plans, About the P.M.’s.
Higher
Trophic Level Working Group. 1998. Draft Report of Higher Trophic Level Working
Group of Florida Bay Program Management Committee, South Florida Ecosystem
restoration Task Force.
Hughes,
D.A.. 1969. Responses to salinity changes as a tidal transport mechanism of
pink shrimp Penaeus
duorarum. Biological
Bulletin 136: 43-53.
Jones,
A.C., D.E. Di itriou, J.J. Ewald and J.H. Tweedy. 1970. Distribution of
early developmental stages of pink shrimp, Penaeus duorarum, in Florida waters. Bulletin of Marine Science 20: 634-661.
Kushlan, J.D., O.L. Bass Jr. and L.C.
McEwan. 1982. Wintering waterfowl in Everglades National Park. South Florida Research Center Report T-670,
Everglades National Park, Homestead FL: 26pp.
Lorenz, J.J. 1997. The effects of
hydrology on resident fishes of the Everglades mangrove zone. National Audubon Society Final Report to
South Florida Research Center, Everglades National Park, Homestead FL: 193pp.
Lorenz,
J.J. In press. The response of fishes to physical-chemical
changes in the mangroves of northeast Florida Bay. Estuaries 1999.
Mazzotti,
F.J. 1983. The ecology of Crocodylus acutus in Florida. Ph.D.
dissertation, The Pennsylvania State University, University Park PA:
161pp.
Mazzotti, F.J. and W.A. Dunson. 1984.
Adaptations of Crocodylus acutus and Alligator for life in saline
water. Comp. Biochem. Physiol. 79A:
641-646.
Mazzotti, F.J. and W.A. Dunson. 1989.
Osmoregulation in crocodilians.
Am. Zool. 29: 903-920.
Mazzotti, F.J., A. Dunbar-Cooper and
J.A. Kushlan. 1988. Desiccation and cryptic nest flooding as
probable causes of embryonic mortality in the American crocodile, Crocodylus
acutus, in Everglades National Park, Florida. Florida Scientist 52: 65-72.
McIvor,
C.C., J.A. Ley and R.D. Bjork.
1994. Changes in freshwater
inflow from the Everglades to Florida Bay including effects on biota and biotic
processes. In: S.M. Davis and J.C. Ogden, eds. Everglades:
The Ecosystem and Its Restoration, St. Lucie Press, Delray
Beach FL: 117-146.
Meeder,
J.F., M.S. Ross, G. Telesnicki, P.L. Ruiz and J.P. Sah. 1996.
Vegetation analysis in the C-111/Taylor Slough Basin. Document 1:
The Southeast Saline Everglades revisited: a half-century of coastal vegetation change. Document 2:
Marine transgression in the Southeast Saline Everglades, Florida: rates, causes and plant sediment
responses. Final Report. Contract C-4244. Southeast Environmental Research Program. Florida International University, Miami FL
Moler,
P.E. 1991. American crocodile population dynamics. Final report, Florida Game and Freshwater Fish Commission,
Tallahassee FL: 23pp.
Morrison,
D. and D.L. Bean. 1997. Benthic macrophyte and invertebrate
distribution and seasonality in the Everglades-Florida Bay ecotone. National Audubon Society Final Report to
South Florida Research Center, Everglades National Park, Homestead FL:
25pp+figures and tables.
Ogden,
J.C. Personal communication. South Florida Water Management District,
West Palm Beach FL.
Ogden,
J.C. 1976. Crocodilian ecology in southern Florida. In:
Research in the Parks:
Transactions of the National Park Centennial Symposium, 1971, National
Park Service Symposium Series No. 1, U.S. Department of the Interior,
Washington D.C.
Ogden,
J.C. 1994. A comparison of wading bird nesting colony dynamics (1931-1946
and 1974-1989) as an indication of ecosystem conditions in the southern
Everglades. In: S.M. Davis and J.C. Ogden, eds. Everglades:
The Ecosystem and Its Restoration.
St. Lucie Press, Delray Beach Fl: 533-570.
Ogden,
J.C., J.A. Kushlan and J.A. Tilmont.
1978. The Food Habits and
Nesting Success of Wood Storks in the Everglades National Park in 1974. Natural Resources Report 16, U.S. National
Park Service, Washington D.C.: 25pp.
Powell,
G.V.N. and R.D. Bjork. 1990. Relationships between hydrologic conditions
and quality and quantity of foraging habitat for roseate spoonbills and other
wading birds in the C-111 basin.
National Audubon Society Second Annual Report to South Florida Research
Center, Everglades National Park, September 1990.
Powell,
G.V.N., R.D. Bjork, J.C. Ogden, R.T. Paul, A.H. Powell and W.B. Robertson,
Jr. 1989. Population trends of some south Florida wading birds. Wilson Bulletin 101: 436-457.
Rice,
J.K. 1997. An analysis of environmental factors influencing juvenile pink
shrimp (Penaeus
duorarum) abundance in southwest Florida. Masters Thesis.
University of Miami, Coral Gables FL.
Schmidt,
T.W. 1993. Community characteristics of dominant forage fishes and decapods
in the Whitewater Bay-Shark River Estuary, Everglades National Park. Technical Report NPS/SEREVER/NRTR-93/12.
Sheridan,
P.F. 1996. Forecasting the fishery for pink shrimp, Penaeus duorarum, on the
Tortugas grounds, Florida. Fishery Bulletin
94: 743-755.
Simmons,
Glen. Personal communication. Homestead FL.
Tabb,
D.C., D.L. Dubrow and A.E. Jones.
1962. Studies on the biology of
the pink shrimp Penaeus duorarum Burkenroad, in Everglades National Park,
Florida. Technical Series 37: 1-30,
Florida State Board of Conservation, University of Miami, Miami Laboratory,
Miami Florida.
Thayer,
G.W., A.B. Powell, and D.E. Hess.
1998. Response of larval,
juvenile and small adult fishes to changes in environmental conditions in
Florida Bay: A decadal comparison.
Proceedings of the 1998 Florida Bay Science Conference, May 12-14, 1998.
Trexler,
J.C., W.F. Loftus, F. Jordan, J.J. Lorenz and J. Chick. In prep.
Empirical assessment of fish introductions in southern Florida: An
evaluation of contrasting views.
Wanless,
H.R., R.W. Parkinson and L.P. Tedesco.
1994. Sea level control on
stability of Everglades wetlands. In: S.M. Davis and J.C. Ogden, eds. Everglades:
The Ecosystem and Its Restoration. St. Lucie Press, Delray
Beach FL: 199-223.
Welsh,
R., M. Remillard and R.F. Doren.
1995. GIS data base development
for south Florida’s national parks and preserves. Photogrammetric Engineering and Remote Sensing 61(1): 1371-1381.