David T. Rudnick, South Florida Water
Management District, West Palm Beach Florida.
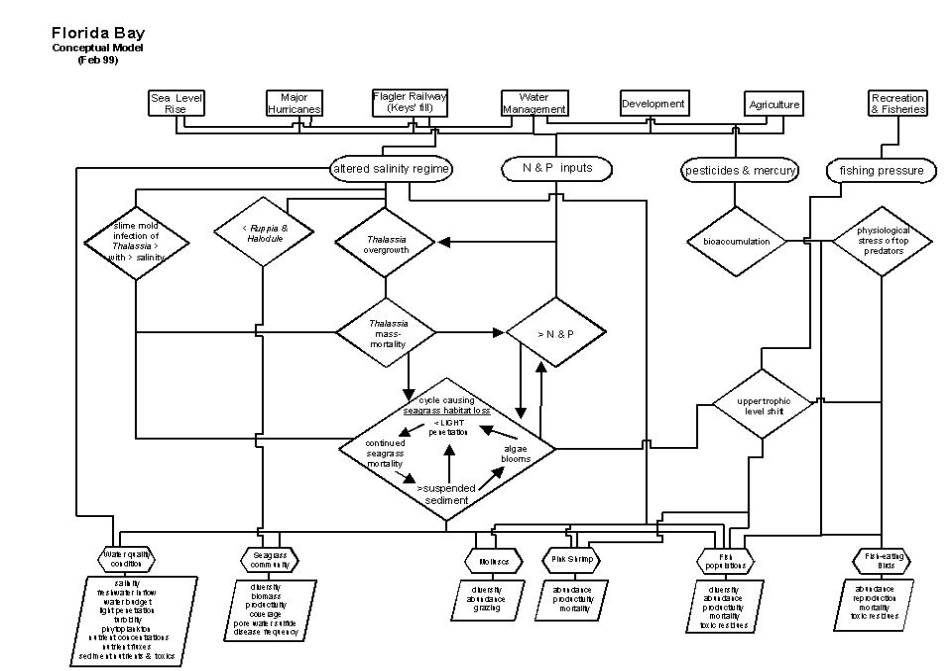
A simple conceptual model of the
Florida Bay ecosystem is presented here.
This model is consistent with our effort to assess the current
understanding of south Florida’s ecosystems, identify the most important human
effects on these ecosystems, identify restoration goals and success criteria,
and identify the minimum measurements required to determine whether these criteria
are being met. The structure of the
model is largely based on the expert opinions of scientists who have focused
their attention on Florida Bay during the past several years. During this time, detailed reviews of our
understanding of the Florida Bay have been presented (Boesch et al. 1993,
Boesch et al. 1995, Boesch et al. 1997, Fourqurean and Robblee 1999). Detailed plans that identify quantitative
information needs for environmental management decision making, as well as
strategies to provide this information, have also been presented (Armentano et
al. 1994, Armentano et al. 1997). While
the conceptual model presented here is largely consistent with the body of
knowledge described in the reviews and plans noted above, some details or
omissions of this model may not be consistent will the opinions of every
contributor listed above or every contributor to the larger Florida Bay
research effort.
Florida Bay primer. Florida Bay is a triangularly shaped
estuary, with an area of about 850 square miles, that lies between the southern
tip of the Florida mainland and the Florida Keys. About 80% of this estuary is within the boundaries of Everglades
National Park. A defining feature of
the bay is it’s shallow depth, with a mean depth of about 1 meter (Schomer and
Drew 1981). This shallowness allows
light to penetrate through the water to the sediment surface in almost all
areas of the bay and results in the potential for the bay to sustain seagrass
beds as a dominant habitat and source of productivity. The shallowness of the bay also affects the
circulation and salinity regime of the bay; with a complex network of shallow
mud banks, water exchange among the bay’s basins and between these basins and
the Gulf of Mexico is restricted (Smith 1994, Wang et al. 1994). With a long residence time and shallow
depth, the salinity of Florida Bay water can rapidly rise during drought
periods. Salinity levels as high as
twice that of seawater have been measured (McIvor et al. 1994). Another defining feature of the bay is that
the sediments are primarily composed of carbonate mud, which can scavenge
inorganic phosphorus from bay waters (DeKanel and Morse 1978).
Until the 1980s, Florida Bay was
perceived by the public and environmental managers as being a healthy estuary,
with clear water, lush seagrass beds, and productive fish and shrimp
populations. By the mid 1980s, however,
catches of pink shrimp had declined dramatically (Browder et al. 1999) and in
1987, the mass mortality of turtle grass (Thalassia) beds began (Robblee et al.
1991). By 1992, the ecosystem appeared
to shift from a clear water system, dominated by primary production on the
sediment (benthic production) to a turbid water system, dominated by algae
blooms in the water column and resuspended sediment. The conceptual model focuses on these changes in seagrasses and
water quality as the central issues to be considered by environmental managers.
Reality check.
The simple model presented below does not address the spatial complexity
of Florida Bay. Florida Bay is, indeed,
not so much a singular estuary, but a complex array of basins, banks, and
islands that differ across a set of regions.
The mosaic of seagrass habitat and mangrove habitat, as well as water
quality and ecosystem processes, vary distinctly with this spatial
variation. Nevertheless, only a single,
generic model is described and this model is intended to summarize the main
characteristics and trends of the bay.
While the structure of this model is appropriate for most areas of the bay,
the relative importance of the model’s components differ considerably among the
bay’s sub-regions. Any application of
this model (for example, recommendations for a specific set of monitoring
parameters and guidelines) must take the spatial variability of the bay into
account.
It has often been assumed that a
direct cause of Florida Bay’s ecological changes is a long-term increase in the
Bay’s salinity that resulted from the diversion of freshwater away from Florida
Bay via SFWMD canals. However, recent
research has indicated that the Bay’s changes are not attributable to a single
cause - while decreased freshwater inflow and resultant increased salinity have
been part of the problem, it appears that other human activities, as well as
natural forces, have also contributed to the problem (Armentano et al. 1997,
Boesch et al. 1993, Boesch et al. 1995, Boesch et al. 1997, Fourqurean and
Robblee 1999). The conceptual model
thus includes both natural and human derived sources of stress (refer to figure
on page D-A-116).
Altered
salinity regime. The salinity regime of an estuary is a
primary determinant of the species composition of communities, as well as
strongly influencing functions of these communities (Sklar and Browder
1998). Salinity is a direct stress on
biota; all estuarine biota have adapted to a given salinity range and a given
degree of salinity variability. For a
given organism, changing salinity beyond this range or too quickly within this
range can result in poor health or death.
Thus long-term changes in salinity level or variability are detrimental to
some species, but favorable for other species.
Florida Bay’s salinity regime varies
greatly over time and space. This
variation ranges from coastal areas that can be nearly fresh during the wet
season, to large areas of the central bay that can have salinity levels near 70
ppt during prolonged droughts, to nearly stable marine conditions (about 35
ppt) on the western boundary of the bay.
The main forces that determine salinity regime in the bay are the inflow
of freshwater from the Everglades, rainfall over the bay, evaporation from the
bay, and exchange with seawater from the Gulf of Mexico and the Atlantic Ocean. Both freshwater inflow and seawater exchange
have changed drastically in the past hundred years, resulting in an alteration
of the bay’s salinity regime.
Freshwater inflow to Florida Bay
decreased in volume and changed in timing and distribution during this century
because of water management.
Hydrologic alteration began in the late 1800s, but accelerated with the
construction of drainage canals by 1920, the Tamiami Trail by 1930, and the
C&SF Project and South Dade Conveyance System from the early 1950s through
1980 (Light and Dineen 1994). With the
diversion of freshwater to the Atlantic coast and Gulf of Mexico coast, the
bay’s mean salinity inevitably increased.
The extent of this increase and how the variability of salinity changed
is not known, but is the subject of current research.
Results from this research indicate
that another important development that altered the salinity regime of Florida
Bay was construction of the Flagler railway across the Keys from 1905 to
1912 (Swart et al. 1996, Swart et al. 1999).
It appears that in the last century, prior to railway construction and
water management, Florida Bay had a lower mean salinity and more frequent
periods of low (10 ppt - 20 ppt) salinity than during this century. The extent and frequency of high salinity
events does not appear to have changed between centuries. The bay’s salinity regime changed abruptly
around 1910 because passes between the Keys were filled to support the
railway. Thus, water exchange between
Florida Bay and the Atlantic Ocean was decreased and water circulation
throughout the bay was probably altered.
Two important natural controls of
salinity, sea level rise and the frequency of major hurricanes
must also be considered. Florida Bay is
a very young estuary, the product of sea level rising over the shallow slope of
the Everglades during the past 4000 years. With rising sea level, the bay not
only became larger but also became deeper.
With greater depth, exchange of water between the sea and the bay probably
increased, resulting in a more stable salinity regime with salinity levels
increasingly similar to the sea.
However, a factor that has counteracted the rising sea is the
accumulation of sediment, which makes the bay more shallow. Most sediment that accumulates in Florida
Bay is carbonate that is precipitated from water by organisms that live in the
Bay. The extent to which these
sediments accumulate is a function of the biology of these organisms, the
chemistry of the water, and the physical energy available to transport these
sediments from the Bay. Major
hurricanes are thought to be important high energy events that can flush
the bay of these sediments. However,
since 1965, no major hurricane has directly affected Florida Bay. Florida Bay’s ecological changes during the
past decade may thus be indirectly influenced by changing circulation patterns
and resultant changing salinity regimes because of changing water depth in the
bay.
Nitrogen
and phosphorus inputs. The productivity and food web structure of
all ecosystems is strongly influenced by patterns of nutrient cycling and the
import and export of these nutrients.
Throughout the world, estuarine ecosystems have undergone dramatic
ecological changes because they have been enriched by nutrients derived from
human activity. These changes have
often been catastrophic, with the loss of seagrasses and the occurrence of
algal blooms and lethal low oxygen or anoxic events. The input of nitrogen and phosphorus (N and P) to estuaries is
thus a potentially important stressor of estuaries.
The importance of N and P as stressors
in Florida Bay is unclear. In general,
the bay is rich in N and poor in P, especially towards the eastern region of
the bay (Boyer et al. 1997). There is
little evidence that nutrient inputs to the bay have increased during this
century, but with expanding agriculture and residential development
in south Florida through this century, and particularly development of the
Keys, nutrient enrichment almost certainly has occurred (Lapointe and Clark
1992, Orem et al. 1998). Anthropogenic
nutrients that enter Florida Bay are derived not only from such local sources
(fertilizer and wastes from agriculture and residential areas), but also from
remote sources. It is likely that
remote contributions to the Gulf of Mexico, such as from the phosphate
fertilizer industry of the Tampa-Port Charlotte area and residential
development from Tampa to Naples, are the most important external sources of
nutients (Rudnick et al. 1999). This
enrichment from external sources, however, may not less important to the bay’s
ecology than it’s own internal sources and cycling. It is, nevertheless, a reasonable hypothesis that a chronic
increase in nutrient inputs has occurred in Florida Bay in this century and
this increase has contributed to ecological changes. Ongoing research will provide information to test this
hypothesis. Development of a water
quality model will also help us understand the effects of past nutrient inputs
and predict the effects of future management scenarios.
In
the conceptual model, water management is listed as a source of stress
because the canal system can transport nutrients through the wetlands toward
the bay, decreasing nutrient retention by the wetlands and possibly increasing
nutrient inputs to the bay. Nutrient
inputs from the Everglades and the Gulf of Mexico are affected not only by
changes of freshwater flowing from Taylor Slough and Shark River Slough, but
also by changes in bay circulation.
Nutrient retention within the Bay is certainly sensitive to these
changes in circulation, which have been caused by Flagler railway
construction and the balance of sea level rise and sedimentation
or sediment removal by major hurricanes. The influence of hurricanes may be particularly important, as
nutrients (particularly P) accumulate in the bay’s carbonate sediment and the
absence of major hurricanes may have resulted in an accumulation of nutrients
during the past few decades.
Pesticides
and mercury. With the widespread agriculture and
residential development of south Florida, the application and release of
pesticides and other toxic materials has increased. Mercury is of particular concern because of high concentrations
of methylmercury in upper trophic level species. However, it is unclear whether anthropogenic mercury inputs to
the Everglades or Florida Bay have increased or whether mercury cycling and
methylation rates have changed.
Pesticides and mercury are of concern because they can affect human
health after the consumption of fish or other biota with high concentrations of
these toxins, and because other species may be adversely affected by these
compounds. To date, there is no
evidence the main ecological changes in Florida Bay are in any way linked to
inputs of toxic compounds. Water
management affects the distribution of these toxic materials and
potentially their transport to Florida Bay.
Controlling water levels in wetlands may also influence the
decomposition of pesticides and mercury methylation rates because both of these
processes are sensitive to the presence of oxygen in soils, which is affected
by water levels.
Fishing
pressure. For any species that is the target of
recreational or commercial fisherman, fishing pressure directly affects
population dynamics and community structure.
Within Everglades National Park, commercial fishing has been prohibited
since 1985, but populations that live outside of ENP boundaries for at least
part of their life cycle, which includes most of Florida Bay’s sport fish
species, are nevertheless affected by fisheries (Tilmant 1989).
A set of Florida Bay’s attributes that
are either indicators of the health of the ecosystem or intrinsically important
to society are given in the conceptual model.
These attributes in most cases are biological components of the
ecosystem, including seagrass, molluscs, shrimp, fish and birds, but also an
aggregated attribute of the chemical and physical condition of the bay, termed
“water quality condition.” While the
list of biological components is broad, it is clear from the links to stressors
that are presented that these attributes are not equally weighted within the
model; the central attribute of this conceptual model of Florida Bay is the
seagrass community. Details of each
attribute and linkage are given below.
Seagrass
community. The keystone of the Florida Bay ecosystem is
its seagrasses (Zieman et al. 1989, Fourqurean and Robblee 1999). These plants are not only a highly
productive foundation of the food web, but are also the main habitat of higher
trophic levels and a controller of the bay’s water quality. Understanding how seagrasses affect water
quality is essential for understanding the current status and fate of the
bay.
Seagrasses affect water quality by
three mechanisms: nutrient uptake and storage, binding of sediments by their
roots, and trapping of particles within their leaf canopy. With the growth of lush seagrass beds, these
mechanisms drive the bay towards a condition of clear water, with low nutrients
for algae growth in the water and low concentrations of suspended sediment in
the water. During the 1970s through the
mid-1980s, lush Thalassia beds grew throughout central and western Florida
Bay and the water was reported to be crystal clear. We hypothesize that with the onset of a Thalassia mass-mortality
event in 1987 (Robblee et al. 1991), these mechanisms reversed, initiating a
cycle that causes continued seagrass habitat loss and propagates
persistent turbid water with algae blooms (Stumpf et al. 1999).
The cause of the 1987 mass-mortality
event is not known, but thought to be related to earlier changes in two
stressors, the salinity regime and nutrient availability. These changes caused Thalassia
beds to grow to an unsustainable density by the mid 1980s. It is also likely that a decrease in shoal
grass and widgeon grass (Halodule and Ruppia) occurred with the Thalassia
increase. Thalassia “overgrowth” may
have occurred because the species thrived when the salinity regime of the bay
was stabilized, with few periods of low salinity. Nutrient enrichment also may have played a role, with a chronic
accumulation of nutrients caused by increased inputs over decades or decreased
outputs because of the absence of major hurricanes or closure of Keys’
passes. The factors that conspired to
initiate the mass-mortality event in 1987 are also unknown, but thought to be
related to the high respiratory demands of the dense grass beds and accumulated
organic matter. During the summer of
1987, with high temperatures, sulfide levels may risen to lethal
concentrations.
Regardless of the cause of the
mass-mortality event, once this event was initiated, the ecology of Florida Bay
changed. The cycle causing continued
seagrass habitat loss, which characterizes the present Florida Bay, is
illustrated in the model. Continued
seagrass mortality results in increased sediment suspension and increased
nutrient release from the sediments (>N & P), stimulating the growth of
algae in the water column. The presence
of both these algae and suspended sediment result in decreased light
penetration to the seagrass bed. In
this cycle, it is this decreased light that stresses the seagrasses and
sustains the feedback loop. Light
penetration is thus an essential aspect of the attribute, water quality.
The dynamics of this feedback loop are
probably not independent of the salinity regime. A disease of seagrass, caused by a slime mold infection, seems to
be more common at salinities near or greater than seawater (≥ 35 ppt) than
at low (15 to 20 ppt) salinities (Landsberg et al. 1996). This may have played a role in either the
initial seagrass mass mortality event, but more likely has served to continue
seagrass mortality since that event.
The incidence of this disease may be directly affected by water
management actions.
If the state of the seagrass community
is to be used as a criterion to decide the success of environmental restoration
efforts, environmental managers must specify the desirability of alternative
states. The consensus among scientists
is that the Florida Bay of the 1970s and early 1980s, with lush Thalassia
and clear water, was probably a temporary and atypical condition. From an ecological perspective, restoration
should probably strive for a more diverse seagrass community, less dominated by
Thalassia
than during that period.
Water
quality condition. Water quality condition reflects not only
obvious characteristics, such as salinity, but also the light field, algae in
the water column, and the availability of nutrients in the ecosystem. All of these characteristics are closely
related to the condition of seagrasses and the food web structure and dynamics
of the bay. While these characteristics
have been monitored and researched since the early 1990s, earlier information
is scarce for salinity and even less available for other characteristics. Thus, at the present time, we do not know
whether nutrient inputs to the bay have actually increased in recent decades or
whether periods with sustained algal blooms and high turbidity occurred in the
past.
Salinity has frequently been suggested
as a primary restoration target.
However, establishing salinity success criteria, such as those used in
the Restudy’s evaluation of the effects of hydrological alternatives on coastal
salinity, depends on the development of a model of the “natural” salinity
distribution of Florida Bay in time and space.
This requires a both a water budget for the bay (monitoring rainfall,
evaporation, and freshwater flow, water level, and salinity) and a hydrodynamic
model, which is now under development.
With modeled salinity variability for a wide variety of target sites in
the bay, the fit of observed salinity fields to modeled fields could serve as
the basis of deciding levels of success.
The magnitude of nutrient inputs to
the bay, and their relationship to freshwater inputs is under
investigation. Success criteria based
on water column nutrient concentrations are probably less meaningful than criteria
based on nutrient loading. Preliminary
results indicate that phosphorus loads to the bay do not greatly increase with
increased freshwater inputs (Rudnick et al. 1999), but Florida Bay is probably
very sensitive to any increase in P availability. Unlike phosphorus, nitrogen loads probably do increase with more
freshwater flow and algae blooms in western and central Florida Bay appear to
be stimulated by increased N (Tomas 1996).
Finally, as emphasized earlier, the
penetration of light through Florida Bay waters is a key to the health of
seagrasses. An important success
criterion should be light penetration, which is largely a function of turbidity
from algae and suspended sediment.
Light penetration should be sufficient to support a viable seagrass
habitat. Such light-based criteria have
been used successfully in other estuaries.
Molluscs.
Because of our ability to assess historical community structure,
molluscs are a good indicators for the entire ecosystem. The composition and activity of the
molluscan community is a function of salinity, seagrass and other habitat
availability, and food supply. Studies
of long-term changes in the composition of this community (by analyzing shells
in the sediment) have indeed found changes that reflect the large-scale changes
of the bay’s salinity regime.
Furthermore, molluscs are likely to be important as grazers of algae in
bay waters; the trophic status of the bay is reflected by molluscan community
composition.
Pink
shrimp. Pink shrimp are intrinsically important to
society as an economic asset. They are
also ecologically important, serving as a major component of the diet of game
fish and wading birds; pink shrimp are an indicator of the bay’s
productivity. Florida Bay and nearby
coastal areas are a primary nursery ground for pink shrimp - a nursery that
supports the shrimp fishery of the Tortugas Grounds (Costello and Allen
1966). Hydrological and ecological
changes in the Everglades and Florida Bay may have impacted this fishery, which
experienced a decline in annual harvest from about 10 million pounds per year
in the 1960s and 1970s to as little as 2 million pounds per year in the late
1980s (Ehrhardt and Legault 1999). This
decline may have been associated with seagrass habitat loss or high salinity
(50 to 70 ppt) during the 1989-1990 drought; experiments have shown that pink
shrimp mortality rates increase with salinities above 40 ppt (Browder et al.
1999). Shrimp harvest statistics
indicate that shrimp productivity increases with increasing freshwater flow
from the Everglades (Browder 1985).
Fish
populations. The health of Florida Bay’s fish populations
is of great importance to the public; the sport fishing is a major economic
asset to the region. It is clear from
recent studies that seagrass beds and the mangrove zone are important habitats
for fish, but no dramatic bay-wide decreases in total fish abundance have been
observed along with seagrass mass-mortality (Thayer et al. 1999). Rather, a shift in the species composition
of this upper trophic level has occurred as a result of the cycle of seagrass
habitat loss and sustained algae blooms.
While some fish species have declined, fish that eat algae in the water,
such as the bay anchovy, are thriving.
Thus the stressors, such as altered salinity, not only affect upper trophic
level animals directly, but also affect them indirectly through food web
changes.
Another important stressor that needs
to be considered with regard to fish populations is the impact of pesticides
and mercury. As concentrations of
mercury and some pesticides greatly increase in upper trophic level animals,
such as sport fish, (via the process of bioaccumulation), and people eat such
fish, a human health issue potentially exists.
Pesticides and mercury can also have ecological impacts by physiologically
stressing organisms (particularly reproductive functions). The extent of any existing problem with
these toxic compounds in Florida Bay is being investigated, but they currently
do not appear to significantly impact human health or ecological health in the
bay. The possible impact of future
restoration efforts on these issues, however, must still be considered.
Among the many fish species that could
be used as indicators of the health of the ecosystem’s upper trophic level,
there is consensus among scientists that spotted sea trout is a key
species. This is the only major sport
fish species that spends its entire life-span in the bay. Population changes and toxic residues in
this species thus reflect the specific problems of the bay and should also
reflect the restoration actions that we take.
For northeastern Florida Bay, the abundance of snook, tarpon, and
crevalle jack should also be considered.
Water
Birds. Florida Bay and its mangrove coastline is an
important feeding ground and breeding ground for water fowl and wading
birds. Conceptual models for other
regions of the Everglades, particularly the mangrove - estuarine transition
zone conceptual model, present more detailed descriptions of the use of bird
populations as ecological indicators and consider a wide variety of birds. For the Florida Bay conceptual model, we
consider only fish-eating birds, such as osprey, brown pelicans, and
cormorants. These birds are important
predators of fish in the bay and are potentially impacted by any stressors that
affect their prey base, including salinity changes, nutrient inputs, toxic
compounds, and fishing pressure. As
with other top predators, these bird species are the most vulnerable members of
the ecosystem with regard to pesticide and mercury effects.
A list of fundamental measures
associated with each of the model’s ecosystem attributes is given. This list should be considered minimal;
interpretation of many of these measures requires a set of associated measures. The list includes not only “structural”
variables (for example, pink shrimp abundance), but also dynamic, process
variables (for example nutrient fluxes).
Note that this list does not reflect the temporal or spatial time scale
at which measurements are necessary, but temporal patterns, such as seasonality
and interannual variability, and spatial patterns are a central aspect of
ecological dynamics. Also note that the
power to predict the fate of any ecosystem requires more than monitoring;
research and modeling are also essential components of sound environmental
management.

Armentano, T., M. Robblee, P. Ortner,
N. Thompson, D. Rudnick, and J. Hunt.
1994. Science Plan for Florida
Bay. 43 pp.
Armentano, T., R. Brock, J. Hunt, W.
Kruzynski, D. Rudnick, S. Traxler, N. Thompson, P. Ortner, K. Cairnes, M.
Robblee, and R. Halley. 1997. Strategic Plan for the Interagency Florida
Bay Science Program. Florida Bay
Program Management Committee, unpublished, 42 pp.
Boesch, D., N. Armstrong, C. D’Elia,
N. Maynard, H. Paerl, and S. Williams. 1993.
Deterioration of the Florida Bay Ecosystem: An Evaluation of the
Scientific Evidence. Report to the
Interagency Working Group on Florida Bay.
Boesch, D., N. Armstrong, J. Cloern,
L. Deegan, R. Perkins, and S. Williams. 1995.
Report of the Florida Bay Science Review Panel on Florida Bay Science
Conference: Report by Principal Investigators, October 17-18, 1995.
Boesch, D., N. Armstrong, J. Cloern,
L. Deegan, S. McCutcheon, R. Perkins, and S. Williams. 1997.
Annual Report of the Florida Bay Science Oversight Panel: Perspectives
from the 1996 Florida Bay Science Conference and Review of the Strategic Plan.
Boyer, J.N., J.W. Fourqurean, and R.D.
Jones. 1997. Spatial characterization
of water quality in Florida Bay and Whitewater Bay by multivariate analysis:
zones of similar influence (ZSI). Estuaries
20:743-758.
Browder, J.A. 1985. Relationship
between pink shrimp production on theTortugas grounds and water flow patterns
in the Florida Everglades. Bull. Mar. Sci. 37:839-856.
Browder, J.A. V.R. Restrepo, J.K.
Rice, M.B. Robblee, and Z. Zein-Eldin.
1999. Environmental influences
on potential recruitment of pink shrimp, Penaeus duorarum, from Florida Bay
nursery grounds. Estuaries (in press).
Costello, T. J., and D. M. Allen.
1966. Migrations and geographic distribution of pink shrimp, Penaeus duorarum,
of the Tortugas and Sanibel grounds, Florida. U.S. Fish Wildl. Serv., Fish.
Bull. 65:449-459.
DeKanel, J. and J.W. Morse. 1978. The
chemistry of orthophosphate uptake from seawater on to calcite and aragonite. Geochem.
Cosmochem. Acta. 42:1335-1340.
Ehrhardt, N.M. and C.M. Legault. 1999.
Pink shrimp recruitment variability as an indicator of Florida Bay
dynamics. Estuaries (in press).
Fourqurean, J.W. and M.B. Robblee.
1999. Florida Bay: a history of recent
ecological changes. Estuaries
(in press).
Landsberg, J.H., B.A. Blakesley, A.
Baker, G. McRae, M. Durako, J. Hall, R. Reese, and J. Styer. 1996.
Examining the correlation between the presence of the slime mold, Labyrinthula
sp.
And the loss of Thalassia testudinum in Florida Bay. In: Proceedings of the 1996 Florida Bay
Science Conference, Key Largo, FL.
Lapointe, B.E. and M.W. Clark. 1992.
Nutrient inputs from the watershed and coastal eutrophication in the
Florida Keys. Estuaries 15:465-476.
Light, S.S. and J.W. Dineen.
1994. Water control in the Everglades:
a historical perspective, p.
47-84. In: S.M. Davis and J.C.
Ogden (eds.), Everglades: The Ecosystem and Its Restoration. St. Lucie Press, Delray Beach, Florida.
McIvor, C.C., J.A. Ley, and R.D.
Bjork. 1994. Changes in freshwater
inflow from the Everglades to Florida Bay including effects on biota and biotic
processes: a review, p. 117-146. In: S.M. Davis and J.C. Ogden (eds.), Everglades:
The Ecosystem and Its Restoration. St.
Lucie Press, Delray Beach, Florida.
Robblee, M.B., T.R. Barber, P.R.
Carlson, Jr., M.J. Durako, J.W. Fourqurean, L.K. Muehlstein, D. Porter, L.A.
Yarbro, R.T. Zieman, and J.C. Zieman. 1991. Mass mortality of the tropical
seagrass Thalassia
testudinum
in Florida Bay (USA). Mar. Ecol. Prog. Ser. 71:297-299.
Rudnick, D.T., Z. Chen, D. Childers,
J. Boyer and T. Fontaine. 1999. Phosphorus and nitrogen inputs to Florida
Bay: the importance of the Everglades watershed. Estuaries (in press).
Schomer, N.S. and R.D. Drew. 1982. An
ecological characterization of the lower Everglades, Florida Bay, and the
Florida Keys. U.S. Fish and Wildlife
Service, Office of Biological Services, Washington DC. WS/OBS-82/58.1. 246 pp.
Sklar, F.H. and J.A. Browder.
1998. Coastal environmental impacts
brought about by alterations of freshwater flow in the Gulf of Mexico. Environ. Management 22:547-562.
Smith, N.P. 1994. Long-term
Gulf-to-Atlantic transport through tidal channels in the Florida Keys. Bull. Mar. Sci. 54:602-609.
Stumpf, R.P., M.L. Frayer, M.J.
Durako, and J.C. Brock. 1999. Variations in water clarity and bottom
albedo in Florida Bay from 1985 to 1997.
Estuaries
(in press)
Swart, P.K., G.F. Healy, R.E. Dodge, P.
Kramer, J.H. Hudson, R.B. Halley, and M.B. Robblee. 1996. The stable oxygen and
carbon isotopic record from a coral growing in Florida Bay: a 160 year record
of climatic and anthropogenic influence. Palaeo,
Palaeo, Palaeo 123:219-237.
Swart, P.K., G.F. Healy, L. Greer, M.
Lutz, A. Saied, D. Anderegg, R.E. Dodge, and D. Rudnick. 1999.
The use of proxy chemical records in coral skeletons to ascertain past
environmental conditions in Florida Bay.
Estuaries
(in press).
Tilmant, J.T. 1989. A history and an overview of recent trends
in the fisheries of Florida Bay. Bull. Mar.
Sci. 44:3-33.
Thayer, G.W., A.B. Powell, and D.E.
Hoss. 1999. Composition of larval, juvenile, and small adult fishes relative
to changes in environmental conditions in Florida Bay. Estuaries (in press).
Tomas, C.R. 1996. The role of nutrients in initiating and
supporting Florida Bay microalgal blooms and primary production. In: Proceedings of the 1996 Florida Bay
Science Conference, Key Largo, FL.
Wang, J.D., J. van de Kreeke, N.
Krishnan, and D. Smith. 1994. Wind and
tide response in Florida Bay. Bull. Mar. Sci. 54: 579-601.
Zieman, J.C., J.W. Fourqurean, and
R.L. Iverson. 1989. Distribution,
abundance and productivity of seagrasses and macroalgae in Florida Bay. Bull. Mar. Sci. 44: 292-311.